As a lot of device manufacturers’ products are under efficacy scrutiny and cost pressure, the role of well-designed clinical trials proving economi...
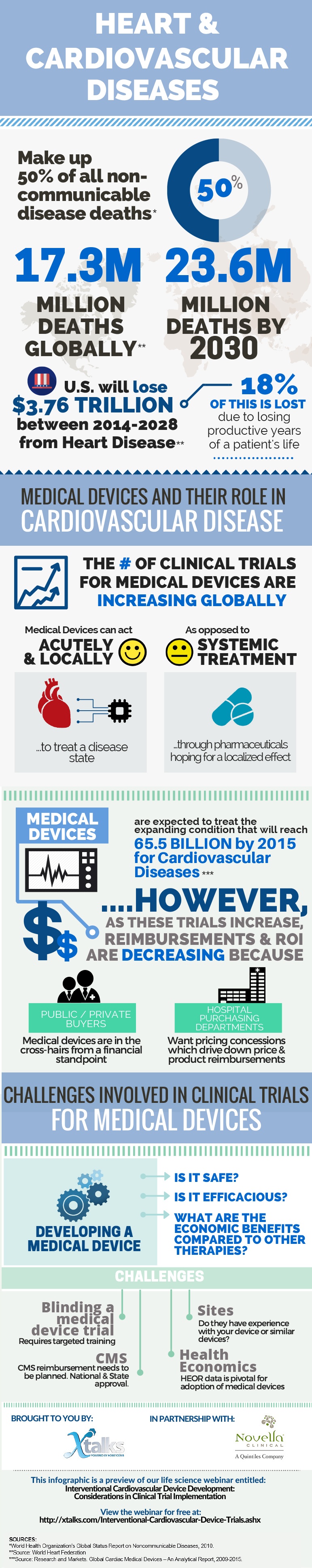

Medical Device Development in Cardiovascular Disease Clinical Trials
An interesting infographic illustrating the larger picture of cardiovascular disease (CVD) progression and the challenges faced by the medical device industry.
At the same time the ability to confirm (or reject) theories connected with the design of a clinical trial depends to a large extent on the adherence of the cohort to the protocol: this is where simulators can play a vital role in aligning the actual intervention with the provided protocol.
Given the available metric data from simulator usage, even corrections to the protocol can be made – e.g. if it is apparent that 40% of a cohort use a device in a different way than the rest of the cohort, research companies may want to either run that group as a subgroup, or adjust the protocol such that the impact is minimized.
Contact Mentice for more information.
Learn more about this Infographic - Medical Device Development in Cardiovascular Disease Clinical Trials and From the Webinar - Interventional Cardiovascular Device Development: Considerations in Clinical Trial Implementation from Xtalks Life Science Webinars.
______________________________________________________________________________________________________________________
{{cta('3ef07bee-e094-48f7-8e81-389e3d8a2eb7')}}
To always stay on top within the field of medical simulation and its development subscribe to our news and resource list
More from Mentice