

This page contains technical information and specifications of our treatment solutions devices, and is intended solely for healthcare professionals.



To ensure that the content you receive is tailored to your region, please select your location from the available options. By doing so, we will be able to provide you with information pertaining to products and services that are specific to your region. This will enable us to better meet your needs and ensure that you receive the best experience possible.
Braided Device Sizing Support Application
Convert 3D DICOM images (3DRA, MRA) or 3D vessel models to extract the vessel morphological description to predict the device foreshortening and apposition at the target zone

Start the simulation by using the threshold segmentation method to create the 3D vessel model from the 3D DICOM image (3DRA/MRA).
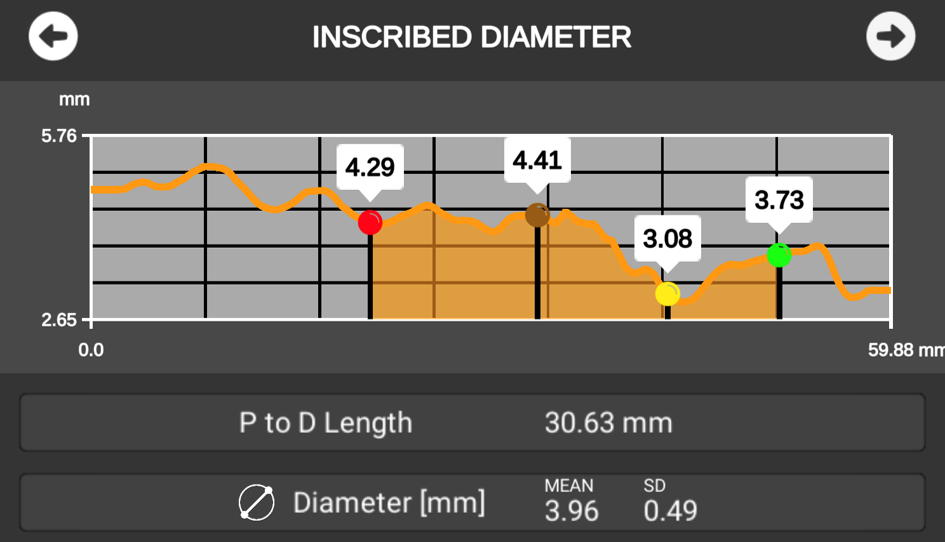
Explore the patient's anatomy in deeper detail: Ankyras measures the morphology considering the 3-dimensional space, providing a detailed analysis of the changing diameter values over the artery, and the segment length to be treated.
Diameter (mm)

Inscribed Diameter (mm)
Area (mm)

Ankyras uses its unique simulation algorithm to assess commercially available flow diverter's final length and position. This method uses device design and detailed morphological descriptors of the treated vessel to show the fitting and position of the device to provide a 95% accurate position and length of the implanted device.


Selecting a flow diverter with good apposition to the vessel wall is possible thanks to the expansion representation over the device surface and the expansion chart. Simulated expansion is colored upon the device's simulated surface, indicating those areas on which the device is fully expanded and not completely attached to the vessel wall based on the device maximum diameter.
Ankyras Porosity characterizes and predicts the local porosity of the flow diverter after implantation. It is intended to help understand differences in local porosity compared to other device brands and sizes and to evaluate the final coverage and expansion in the desired region.


Simultaneously simulate multiple flow diverters to assess whether a telescoping technique suits the patient-specific anatomy.
The different Ankyras platforms allow the use of the software in the user’s most suitable way; from preparing a simulation offline and checking the results in site, to preparing a simulation online and sharing it with other Ankyras members or third parties, who will be able to access the shared simulation as well with limited functionalities.


Local software installed on your computer
Require internet:
View Simulation:
.svg)
Create & edit simulation:
.svg)
Share Simulation:

Login to Ankyras Online in your web browser
Require internet:
.svg)
View Simulation:
.svg)
Create & edit simulation:
.svg)
Share Simulation:
.svg)

Mobile app installed on your smartphone or tablet
Require internet:
.svg)
View Simulation:
.svg)
Create & edit simulation:
Share Simulation:
.svg)
Each platform provides different approaches, ranging from pure visualization to full workflow control, enabling the workflow to run before the intervention with diagnostic 3D images, which can be brought to the operating room using standard mobile devices.


We are here to guide and help you find the solution that is the best fit for your unique needs. Fill in the form to connect with us and a suitable solution expert will get back to you as soon as possible

Rambla Catalunya 53, 4-H, 08007, Barcelona, Spain.
Disclaimer & Regulatory Information
Ankyras is manufactured by Mentice Spain S.L. and distributed by Mentice subsidiaries and partners in approved markets.
Mentice Spain SL: Mentice Spain SL has obtained the ISO 13485 certification with number MD 784027
Ankyras certifications: The Ankyras product complies with the Medical Device Regulation (EU CE Mark, MDR 784032) and the UK Conformity Assessed (UKCA 784033)